
We can find Aluminium in the homes we live in, cars, trains, aeroplanes, mobile phones, computers, and other daily stuffs in modern interior designs. Aluminium is three times lighter than iron, easy in processing, durable almost as much as steel, and corrosion-resistant. But, not many people realized that Bauxite is used today as the primary raw material in Aluminium production.
Bauxite does not have a specific composition, it is a sedimentary rock with a relatively high Aluminium content. It is the world's main source of Aluminium. Bauxite consists mostly of the Aluminium minerals gibbsite (Al(OH)3), boehmite (γ-AlO(OH)) and diaspore (α-AlO(OH)), mixed with the two iron oxides goethite and haematite, the Aluminium clay mineral kaolinite and small amounts of anatase (TiO2) and ilmenite (FeTiO3 or FeO.TiO2).
Beside Aluminium, Bauxite is used in a lot of industries like the chemical industry, refractory, abrasive, cement, steel, and petrol industry amongst others. In chemical, Bauxite along with Alumina is used in the manufacturing of Aluminium chemicals.
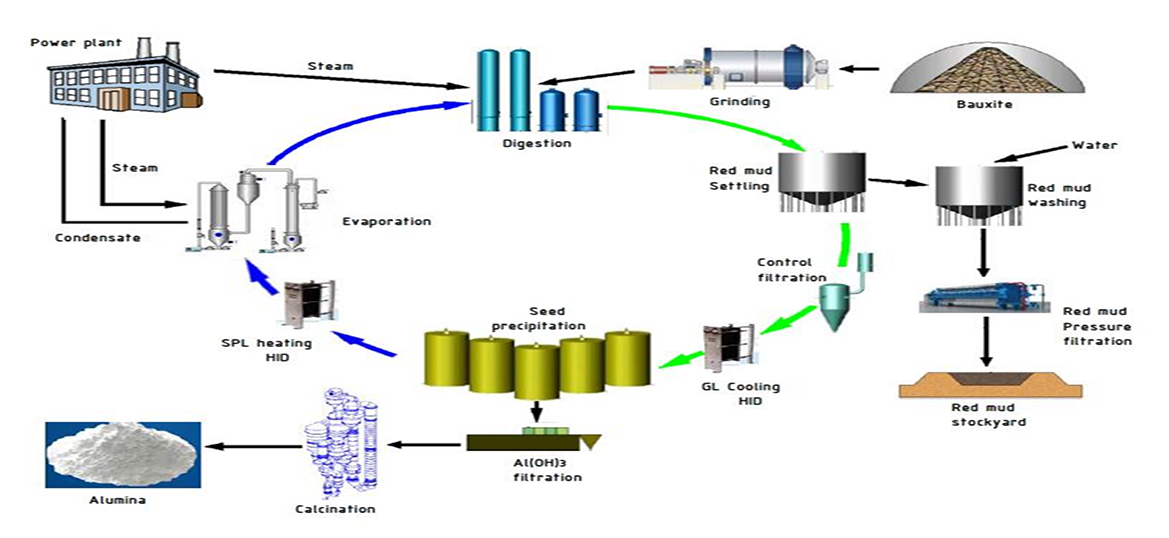
In the first stage of Aluminium production Bauxite is processed, crush the Bauxite and purify it using the Bayer Process into Alumina, or Aluminium oxide Al2O3. In the Bayer Process, the Bauxite is washed in a hot solution of sodium hydroxide, which leaches aluminum from the Bauxite. Alumina looks like white powder and it is then processed into Aluminium at Aluminium smelters using electrolytic reduction.
The Bayer process was invented in 1887 by Carl Josef Bayer. The Austrian chemist sought to develop a method for supplying Alumina to the textile industry (for use as a mordant, a substance that combines with a dye and thereby sets the color in a material). The Bayer process gained importance in the aluminum production industry when combined with the Hall–Héroult electrolytic process. With the two processes combined, Bauxite ore can be processed into Alumina, which is then converted into aluminum. Today, the Bayer process is virtually unchanged and is used to produce nearly all of the world’s Alumina supply, as an intermediate step in aluminum production. The process stages are milling, desilication, digestion, clarification/settling, precipitation, evaporation, classification, and finally calcination.
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididun
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididun
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididun
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididun


As explained above, Bauxite is the raw material for the production of aluminum. Depending on the quality of the ore, anywhere between two to three tons of Bauxite is required for each ton of Alumina produced. With years of INALUM’s Aluminium smelting and ANTAM’s mining expertise, we will be a part of world’s leader in the development, manufacture and supply of premium Smelter Grade Alumina product.

Orchestrating passion, long-existing technical knowledge, continuous improvement and caring is our “horse-power” of growth and achieving sustainable economic. As a part of worldwide society, we believe that driving innovation and eco-friendly thinking in every aspect of our business, services, processes, and products will affect not only for our sustainable business, but also for societal activities.